Astrobiology Revealed #6: Ana Franco
on Boron and the origins of life
by Aubrey Zerkle
This week we asked Ana Franco, Chemistry PhD student in Instituto Superior Técnico at Universidade de Lisboa, about her recent hypothesis paper “Boron as a Hypothetical Participant in the Prebiological Enantiomeric Enrichment”. Astrobiologists seek to unravel how life emerged on our planet to illuminate how life might originate on other worlds. In this Q&A, Ana discusses one of the outstanding mysteries of life’s origins and how she thinks boron could solve it. (This interview has been edited for length and clarity.)
How did you become interested in origins of life research?
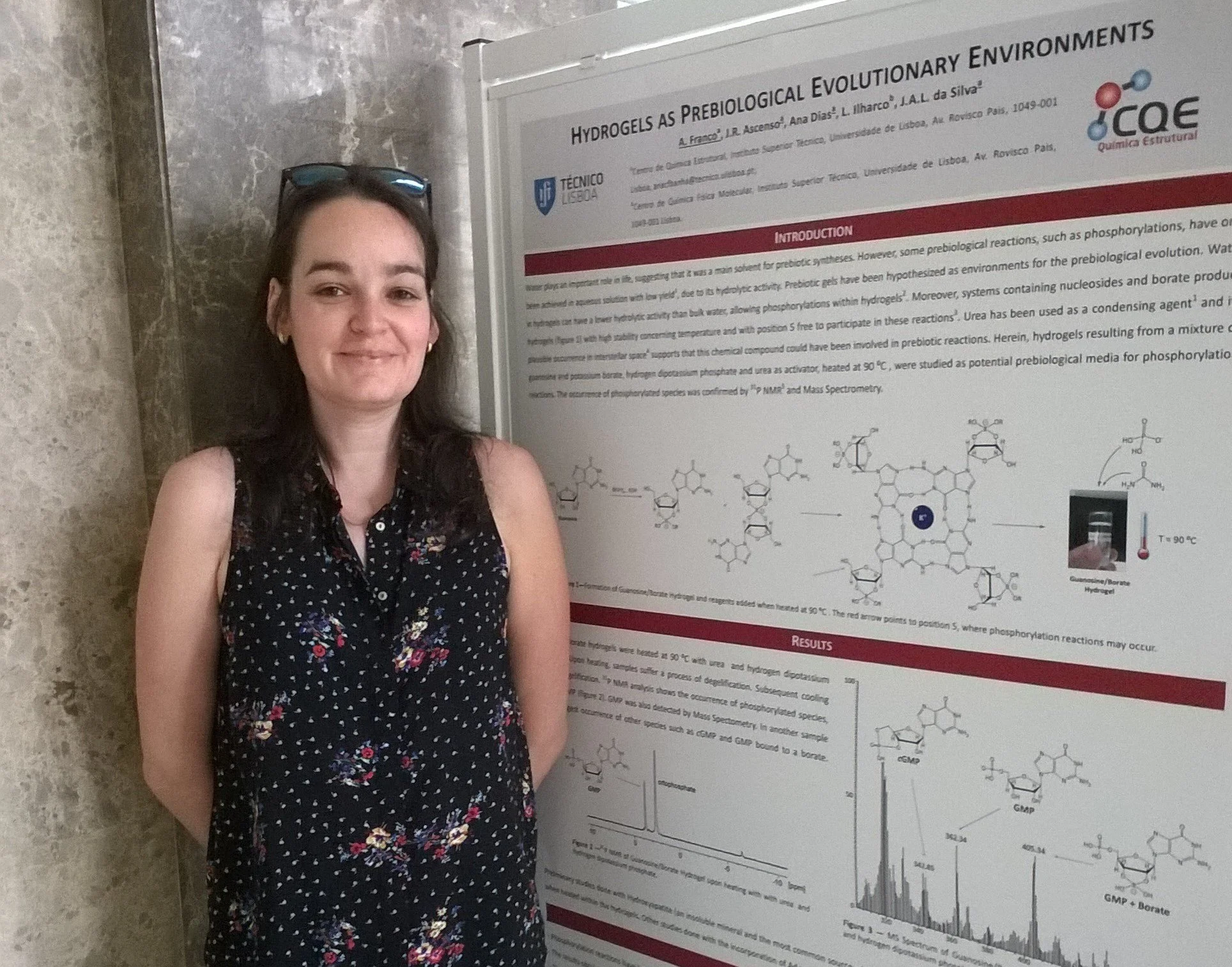
It happened by chance, really. I worked a bit on hydrogels and aerogels during my Masters and I’d worked with nanoparticles before, so at the time I was more into materials science than anything else. At least, I thought that was what my thesis would be about. But there was a bioinorganic chemistry course that really piqued my interest, and I remember particularly enjoying a couple of classes on prebiotic chemistry and the origin of life. I read a few papers on the subject and thought it was intriguing.
When it was time to choose the topic for my thesis, my bioinorganic Professor (who later became my supervisor) showed me a couple of hypothesis papers that proposed life could have started inside a hydrogel. I thought it was such an original idea and I remember getting very excited and thinking about it nonstop. The idea of applying what I’d learned about gels to something completely different than what I was used to seemed too good an opportunity to pass up. And I really thought someone should go into a lab and experiment and try to prove these hypotheses right. So, why not me?
Of course, not everything was easy, and it was scary at first. I knew more than enough about hydrogels, but not much about prebiotic chemistry. Sometimes it felt like I’d taken an unnecessary risk. It is a difficult field because it’s knowledge for the sake of knowledge, with no real application. But the more I studied and the more I learned about it, the more I fell in love with it. And it’s nice when you get to enjoy your work, right?
There are many fascinating questions about life’s beginnings – which of these mysteries were you addressing with your recent paper in Astrobiology?
We were focusing on the emergence of homochirality. Certain molecules, such as sugars and most amino acids, are what we call chiral. Simply put, every time we synthesize a chiral molecule in a lab we get two versions of that molecule, where one is the mirror image of the other. We say one molecule is right-handed and the other is left-handed, and no matter how hard we rotate it, we can’t superimpose them. We call these two versions of the same molecule enantiomers. Unless we use a catalyst, a solvent or a chiral agent to tip the scales, we’ll always get equal amounts of both enantiomers. Consequently, we’d expect the same to happen in nature.
Curiously, it doesn’t. Most amino acids produced by living organisms are left-handed, and the sugars that form nucleic acids, like RNA and DNA, are right-handed. Hence the name, homochirality. So where did all of this [homochirality in life] come from? For this to happen, there must have been some kind of process or mechanism that tipped the scales towards the synthesis of chiral molecules in a prebiological environment. Something made right-handed sugars more abundant than left-handed ones before life itself began. And the opposite happened with amino acids. It's an interesting and fascinating question.
How do you propose boron could help solve this problem?
In two ways: either as a constituent of minerals or of hydrogels. For the first, it’s important to mention that, like molecules, crystals and minerals can also be chiral or have chiral surfaces. It’s also well known that chiral mineral surfaces are very common in nature. For example, common minerals like quartz, olivine and pyrite have them. And right-handed mineral surfaces adsorb only right-handed molecules, while left-handed surfaces adsorb only left-handed molecules. Minerals can and have been used as catalysts in the selection and synthesis of enantiomers, which suggests they could have had an important role in the origin of homochirality.
But why boron, specifically? Well, recent studies suggest that boron inorganic species, such as borate and boric acid, may have been associated with the prebiological synthesis of ribose (a sugar), ribonucleosides and ribonucleotides, which are RNA’s building blocks. And all of these molecules are right-handed. Moreover, it is plausible that boron minerals were present in prebiological environments. If they had chiral surfaces, which is extremely likely, they may have catalysed the homochiral enrichment of these right-handed building blocks.
The same approach can be used towards hydrogels, which are 3-D structures that self-assemble in natural rocky watery environments. As stated before, they have been previously proposed as frameworks for prebiological reactions. Moreover, some ribonucleosides form hydrogels with borate and other metals. For example, guanosine self-assembles into a hydrogel in the presence of borate and potassium or sodium.
Now, guanosine is a chiral molecule, so it has two enantiomers. Each enantiomer self-assembles into a hydrogel, but each has different structure, properties, and reactivity. So, imagine that, in the right conditions, both enantiomers self-assemble into hydrogels, but the right-handed guanosine hydrogel is more stable. For example, it could have greater thermal resistance or re-assemble faster after destruction. This way it would have an advantage over left-handed guanosine, which is not present in RNA.
Thus, boron minerals with right-handed crystal surfaces or right-handed guanosine hydrogels could have solved the problem of homochirality. Or, given that hydrogels form in rocky watery environments, maybe it was a bit of both.
You mentioned in your paper that boron is relatively rare in Earth's crust. What types of environments does boron accumulate in? Does this have implications for environments where life might have originated?
That’s right, boron is not very abundant in Earth’s crust, but it is the eleventh most abundant element in seawater. Large quantities tend to concentrate in secondary sedimentary deposits of boron minerals, usually formed in closed basins and under arid conditions. Or in other words, dry lakes. These deposits result from weathering of primary minerals, mainly formed by volcanic activity, like hydrothermal fluids and thermal springs.
It has been shown many times that wet/dry cycles between aqueous and dry environments promote and improve the yield of ribonucleotide formation, which is an RNA building block. This type of reaction could have happened in dry lakes, periodically covered by water that could have slowly evaporated into the atmosphere. It is possible that an early primitive continental crust could have already been formed on a prebiological Earth, making this type of environment a likely scenario. And these are the type of environments in which boron minerals are formed, hence where life could have originated.
You also mentioned the RNA World hypothesis is currently the most widely accepted explanation for the origin of life. How does the boron hypothesis support or challenge that model?
I think it supports it in many ways. The RNA World hypothesis assumes that RNA was formed before DNA and proteins, since it is an auto-catalytic molecule that can replicate itself and store information. However, it has certain issues. For example, ribose is produced in low yields under prebiological conditions. In a prebiological environment, this would have affected its long-term accumulation and subsequent incorporation into larger biological molecules, such as RNA’s building blocks. It is also easily degraded. Boron, in the form of borate or boric acid, binds with ribose, stabilizing it within a reasonable range of pH and temperature, therefore impeding its degradation.
Another issue is that, in aqueous solution, ribose occurs in two different conformations: 80% is in the form of ribopyranose, a six-membered ring, and 20% is as ribofuranose, a five-membered ring. The same doesn’t happen when borate is present in solution. It binds to ribose, inducing only the ribofuranose conformation, which is the sole conformation present in RNA.
Another interesting fact is that borate binds to ribose the right way. To have a ribonucleotide, two specific positions of this sugar must react with a phosphate group and a nucleobase, either guanine, adenine, cytosine, or uracil. Borate binds to ribose, leaving these two positions free, promoting selectivity, and preventing unwanted side reactions.
Aside from calcium, which stabilizes ribose in the solid state, it’s the only species that does all this. Therefore, I’d say boron is a very important element in support of the RNA World hypothesis.
And since this is an astrobiology-based blog, I have to ask – what do you think is the significance of boron being found on Mars, in terms of early life on Earth and the potential for past or present life on Mars?
Well, it should be stressed that the oldest known boron minerals [on Earth] are primary metamorphic tourmalines, such as dravite and schorl, formed around 3.6 billion years ago. In other words, not old enough [for the origin of life]. In fact, none of the boron minerals known today seem to have been available on early Earth. However, there is no reason to dismiss the existence of earlier boron minerals. It is also possible that not all primitive boron minerals are known. In 2015, a study suggested that approximately 25% of boron minerals remained to be discovered.
So, it's very important that the boron evaporites detected on Mars are older than all the boron evaporites found on Earth. Because if these boron evaporites were formed on [early] Mars, it suggests that boron-concentrating processes could have also occurred on early Earth. Even though the geological processes could have been different than today, if there was a continental crust, boron minerals could have been formed in arid lacustrine environments. In the presence of the right reagents and in the right conditions, life could have emerged on early Earth.
What does this tell us about Mars? I think it’s important to gather more data to see if this type of scenario could have been possible on this planet.
Do you have any plans for follow-up work to further investigate the potential role of boron in prebiotic chemistry?
Sometimes I wish I could travel around the world and dig for new boron minerals, but that would be completely out of my field and I’m sure I wouldn’t know how to do it. But there are many things to do in a lab regarding boron and homochirality, particularly regarding hydrogels.
Besides that, boron seems to have been an important element in the prebiological synthesis of ribonucleosides and ribonucleotides. Was it also important in the synthesis of RNA? What about other important biological molecules? I think these are all questions worth exploring.
Finally, is there anything else you’d like to mention that I haven’t asked about?
Yes, I’d like to thank my co-authors, Professor José Armando da Silva and Professor Maria Orquídia Neves, for all the help. Compiling information on more than three hundred boron minerals wasn’t easy, but somehow we managed to do it.